Stainless steel passivation is a vital process that significantly contributes to the metal’s durability and versatility. By understanding the properties of stainless steel and the importance of passivation, industries can ensure the longevity and reliability of their stainless steel components.
What is stainless steel, and what are its properties?
Stainless steel is a remarkable alloy known for its resistance to corrosion and rust, which it owes to the addition of chromium. When added to iron, chromium reacts with oxygen to form a protective layer of chromium oxide, which is only one to three nanometers thick but provides significant protection against corrosion. This inherent resistance to corrosion makes stainless steel an ideal material for many applications, from kitchen utensils to medical devices and industrial equipment.
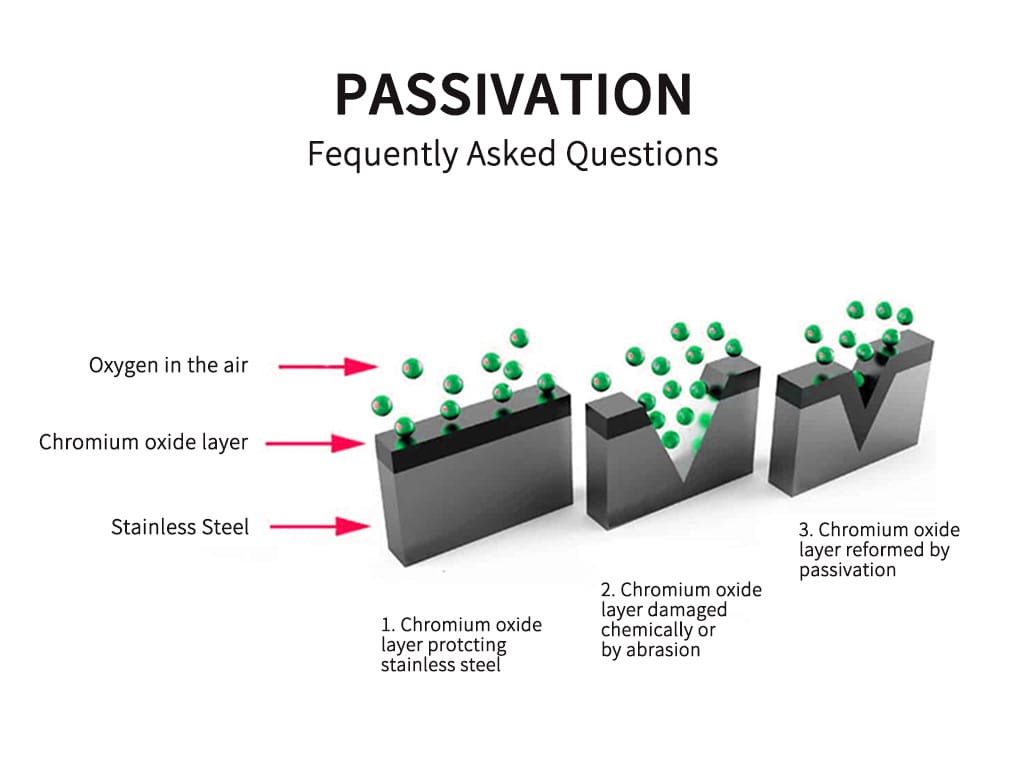
Why is passivation necessary for stainless steel?
Despite its natural corrosion resistance, stainless steel can still be susceptible to surface contamination. This contamination can occur during manufacturing processes, such as machining or welding, where free iron particles may be deposited on the surface. These contaminants can act as initiation sites for corrosion, compromising the integrity and appearance of the stainless steel. Passivation is a post-fabrication process that enhances the natural corrosion resistance of stainless steel by removing these contaminants and promoting the formation of the protective chromium oxide layer.
What is the process of passivation for stainless steel?
Passivation involves treating stainless steel with an acid solution to remove free iron from the surface. Historically, nitric acid has been the primary agent used for this purpose, but citric acid is becoming increasingly popular due to its safety and environmental benefits. The process typically includes thoroughly cleaning the stainless steel to remove oils, greases, and other contaminants. After cleaning, the stainless steel is immersed in an acid bath, which reacts with the iron to enhance the protective oxide layer. This layer is invisible and extremely thin, yet it effectively prevents oxygen and moisture from reaching the underlying iron, thus preventing rust and corrosion.
The passivation process enhances stainless steel’s corrosion resistance and improves its durability and versatility. By ensuring a clean and robust passive layer, stainless steel components can maintain their integrity and functionality for longer, reducing the need for frequent maintenance and replacement. This makes passivation a critical step for industries that demand high standards of cleanliness and durability, such as pharmaceuticals, food processing, and aerospace.
How Passivation Works to Protect Stainless Steel
Passivation is a chemical process that enhances stainless steel’s natural corrosion resistance. The process works by removing free iron and other contaminants from the steel’s surface, which can act as initiation sites for corrosion. When these contaminants are removed, the chromium in the stainless steel reacts with oxygen in the air to form a thin, protective chromium oxide layer. This layer is only a few nanometers thick but is highly effective at preventing further oxidation of the underlying metal, thus protecting the steel from corrosion.
The Role of Chromium in Passivation
Chromium plays a crucial role in the passivation of stainless steel. The element reacts with oxygen to form the passive chromium oxide layer that shields the steel from corrosion. This layer is self-repairing; if it is damaged or removed, the chromium in the steel will react with oxygen to rebuild the oxide layer, provided sufficient oxygen is available. Chromium also enhances the steel’s resistance to various forms of localized corrosion, such as pitting and crevice corrosion, and improves its performance in oxidizing and acid-chloride environments.
Comparing Nitric Acid and Citric Acid Passivation Methods

Two primary acids are used in the passivation process: nitric acid and citric acid. Nitric acid has been the traditional choice for passivation, as it effectively removes iron and other contaminants from the surface of stainless steel. However, citric acid is gaining popularity due to its safety, environmental benefits, and suitability for food and beverage processing. Citric acid passivation is also less aggressive and can be a better choice for specific stainless steel grades that might be damaged by nitric acid.
Both acids work by reacting with the free iron on the surface of the steel, but they do so in different ways and at different rates. Nitric acid treatments can also include sodium dichromate to promote faster oxidation, especially for less resistant steel grades. The choice between nitric and citric acid will depend on the specific requirements of the stainless steel grade and the desired outcome of the passivation process.
It’s important to note that the passivation process parameters, such as the total time of submersion, temperature, and chemical concentration, can vary significantly depending on the steel grade and the chosen acid. Additionally, proper pre-cleaning of the steel is essential to ensure the effectiveness of the passivation process.
The Passivation Procedure

1. Cleaning: The First Critical Step
The passivation process begins with a thorough cleaning of the stainless steel surface. This step is essential to remove any oils, greases, and other contaminants that could interfere with the passivation process. Standard cleaning methods include commercial degreasers or cleansers to remove machining oils or coolants. In some cases, more stubborn contaminants like thermal oxides may need to be extracted by grinding or acid pickling. The effectiveness of the cleaning step is often validated through tests such as the camphor test.
2. The Acid Bath: Nitric vs. Citric Acid
After cleaning, the stainless steel is immersed in an acid bath, which is the core of the passivation process. The two most common acids used are nitric acid and citric acid. Nitric acid is a vital mineral that quickly dissolves iron compounds and other trace metals on the surface, activating the chromium oxide layer. Citric acid, conversely, is safer to use, biodegradable, and effective at removing iron without the disposal problems and safety concerns associated with nitric acid. The choice between the two acids often depends on the specific requirements of the stainless steel grade, environmental considerations, and the desired outcome of the passivation process.
3. Testing and Certification Post-Passivation
The final step in the passivation process is quality testing to ensure the chemical treatment succeeded. This testing often involves evaluating the surface of passivated parts for the presence of free iron, which can be done using test kits from chemical supply firms. The industry standards for passivation, such as ASTM A967 and AMS 2700, provide testing methods and acceptance criteria guidelines. These standards are highly standardized due to the importance of passivation in enhancing the corrosion resistance and longevity of stainless steel parts.
Steps-by-Step Breakdown to Passivate Stainless Steel
Passivation is a critical process that enhances the corrosion resistance of stainless steel. Here’s a step-by-step guide on how to passivate stainless steel:
Degrease Parts: The first step in the passivation process is thoroughly cleaning the stainless steel parts. This involves removing contaminants such as dust, metal chips, and cutting oils. A degreaser is typically used to remove these contaminants effectively.
Water Rinse: After degreasing, the parts are rinsed with water to remove residual cleaning agents. This ensures that the surface is immaculate before the passivation process begins.
Acid Bath Immersion: The cleaned parts are then immersed in an acid bath, typically of nitric or citric acid. This acid bath removes free iron and other surface contaminants from the stainless steel, which is crucial for forming the protective chromium oxide layer.
Rinse Again: After the acid bath, the parts are rinsed with water to remove residual acid.
Neutralize (Optional): Some passivation processes include A neutralization step after the acid bath. This involves immersing the parts in a neutralizing solution to balance the pH and ensure no residual acid remains on the surface.
Dry: The final step in the passivation process is drying the parts. This can be done using air, oven, or other suitable methods. Drying is essential to prevent water spots or stains that could lead to corrosion.
Test: After the passivation process, it’s essential to test the stainless steel surface to ensure its effectiveness. This can involve various methods to measure the corrosion resistance of the passivated layer.
Dos and Don’ts of Passivating Stainless Steel
Dos
Design for Passivation: When designing parts, equipment, and systems, avoid areas where dirt or cleaning solutions might get trapped. This can help ensure a more effective passivation process.
Clean Before Passivation: Ensure the steel part is properly cleaned before starting. This includes pre-cleaning, degreasing, and de-scaling before passivation.
Treat Weld Areas: Weld areas should receive special attention during passivation. These areas can often harbor contaminants that need to be removed.
Use Dedicated Equipment: Assign specific machines to fabricate stainless steels only and stay with the same preferred coolant to cut stainless steels, excluding all other metals.
Rack Parts Individually: Rack parts individually for treatment to avoid metal-to-metal contact. This is especially important with free-machining stainless steels, where the free flow of passivating and rinse solutions is needed to diffuse away corrosion products from sulfides and avoid pockets of acid.
Use Dechlorinated Water: Use dechlorinated water for rinsing whenever possible. This can help prevent the introduction of additional contaminants.
Replace Acid Baths: Regularly replace the acid baths to ensure proper passivation. Over time, the acid can become contaminated or lose its effectiveness.
Implement a Racking Storage System: Implement a racking storage system that avoids metal-to-metal contact. This can help prevent the introduction of additional contaminants.
Don’ts
Avoid Passivating Certain Parts: Don’t passivate stainless steel parts that have been carburized or nitrided. Parts so treated may have their corrosion resistance lowered to the point where they are subject to attack in the passivating tank.
Avoid Certain Tooling: Don’t use tooling with iron content in a shop environment that is not exceptionally clean. Steel grit can be avoided by using carbide or ceramic tools.
Avoid Improper Heat Treatment: Don’t forget that an attack can occur in a passivating bath if parts are improperly heat-treated. High-carbon, high-chromium martensitic grades must be hardened to become corrosion-resistant.
Avoid Leaving Cutting Fluids on Parts: Avoid cutting fluids on parts before heat treating. If cutting fluids remain on bright, hardened parts, surface carburization may occur, leading to a loss of corrosion resistance.
Avoid Certain Cleaning Agents: Don’t use specific cleaning agents that can harm stainless steel. Some agents can cause excessive oxidation or damage the protective chromium oxide layer.
Grades of Passivated Stainless Steel
Stainless steel comes in various grades, each with unique properties and applications. The passivation process can be applied to all these grades to enhance their corrosion resistance. Here are the main categories of stainless steel that can be passivated:

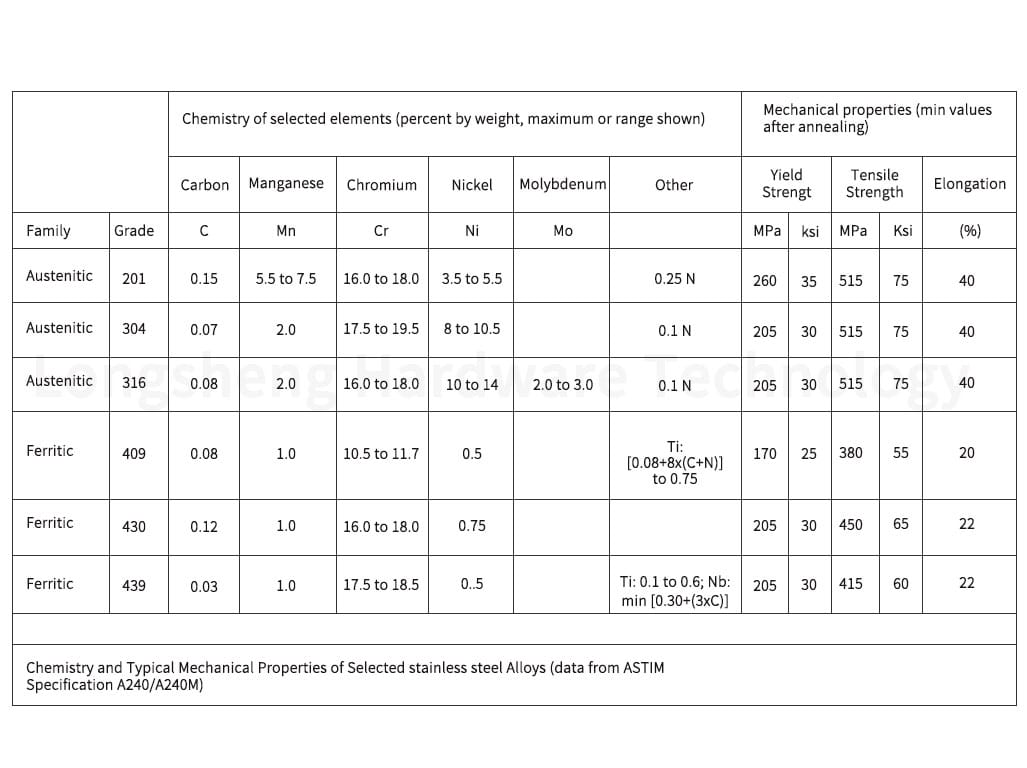
Austenitic Grades: This category includes all 200 and 300-series stainless steels. Austenitic stainless steels are known for their superior corrosion resistance and are commonly used in various applications. They can be effectively passivated using both nitric and citric acid treatments.
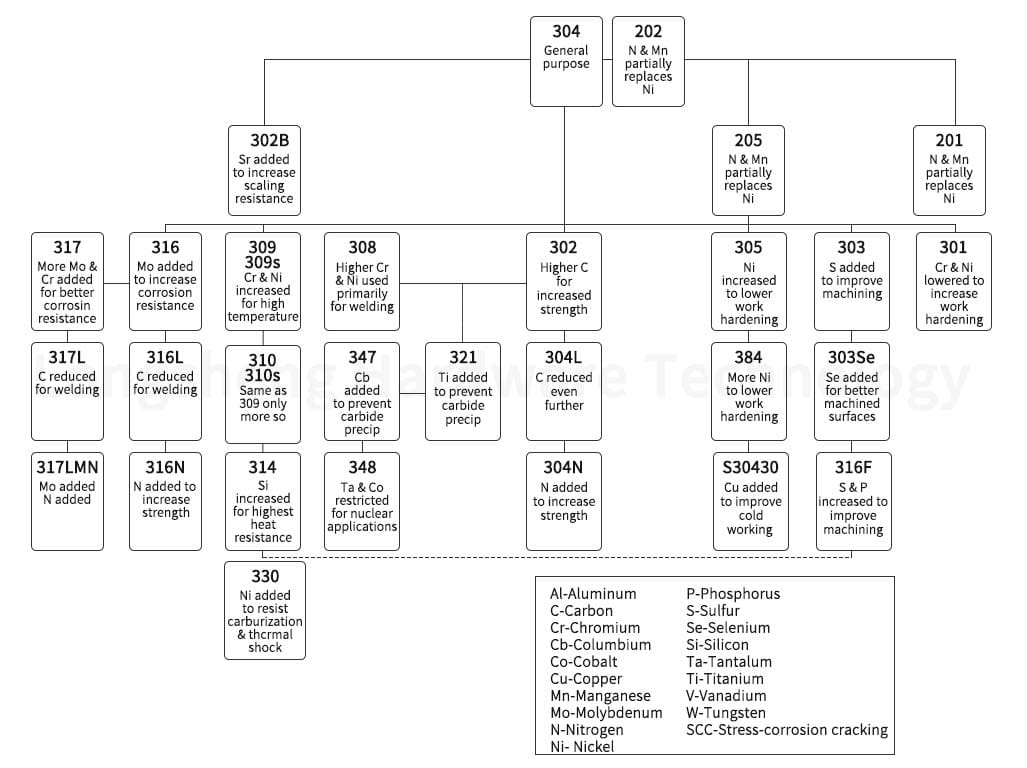
Ferritic Grades are part of the 400 series and are low-carbon alloys. Ferritic stainless steels are magnetic and have good corrosion resistance and formability. They can also be passivated using nitric and citric acid treatments.
Martensitic Grades are also part of the 400 series but are higher carbon alloys. Martensitic stainless steels are known for their high strength and hardness. They can be passivated, but the process may be more complex due to their high carbon content.
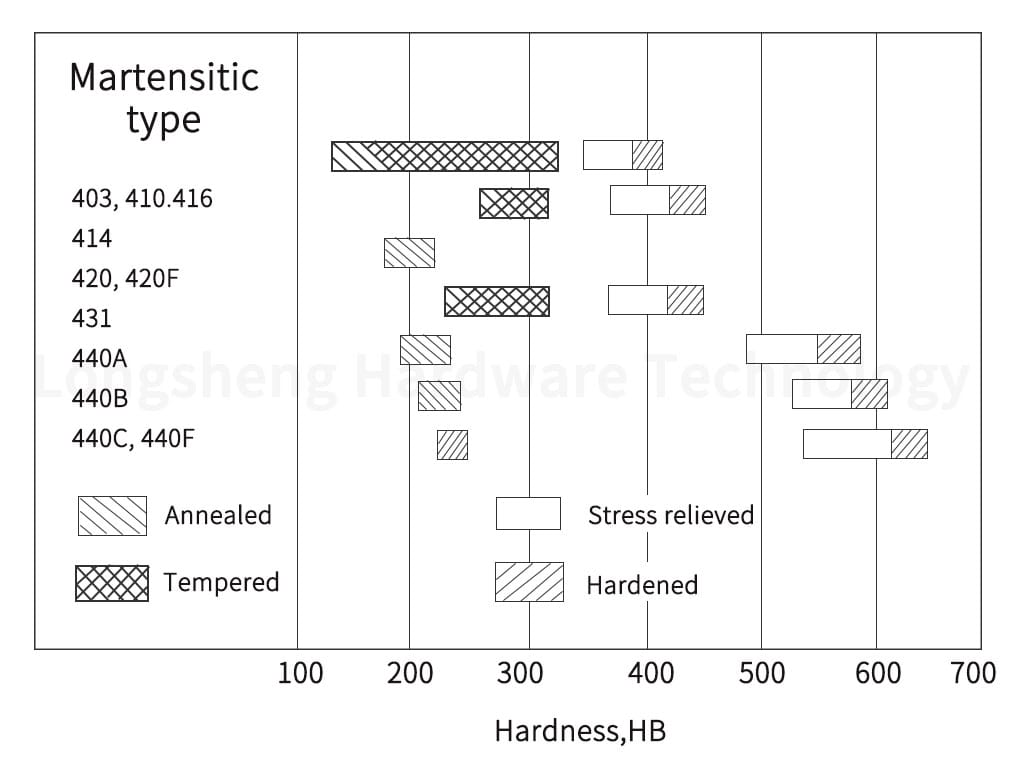
Medical & Exotic Alloys: This category includes alloys like Titanium, MP35N, 316LVM, Cobalt-Chromium, Inconel, Nickel-Alloys (Nickel 200), Kovar, and Invar. Due to their high strength and corrosion resistance, these alloys are often used in medical and aerospace applications. They can be passivated, but the process may require specialized treatments.

Each grade’s passivation process involves cleaning the surface, immersing the parts in an acid bath, and thoroughly rinsing and drying them. The specific process parameters, such as the type of acid used and the duration of the acid bath, can vary depending on the grade of stainless steel and the application’s specific requirements.
It’s important to note that while passivation enhances stainless steel’s corrosion resistance, it does not make the material completely corrosion-proof. The degree of corrosion resistance will depend on the alloying composition, heat treatment, internal stresses, and the effectiveness of the passivation treatment. Regular maintenance and re-passivation may be necessary, especially for parts exposed to harsh environments or high chloride content.
Benefits of Stainless Steel Passivation
1. Enhanced Corrosion Resistance and Durability
Passivation significantly enhances the corrosion resistance of stainless steel by removing surface contaminants and promoting the formation of a passive oxide layer. This process increases the ratio of stable chromium atoms to the more reactive iron atoms on the metal’s surface, which is crucial for the material’s ability to resist corrosion. By doing so, passivation ensures the durability and longevity of stainless steel, safeguarding it from the harsh effects of corrosion and other environmental factors. Regular passivation treatments can lead to less frequent and shorter system shutdowns, thereby reducing the risk of unplanned downtime and boosting revenue due to increased uptime.
2. Improved Aesthetics and Surface Cleanliness
The passivation process prevents corrosion and improves the appearance of stainless steel, giving it a brighter and more attractive look. By removing free iron and other contaminants from the surface, passivation ensures a clean, uncontaminated surface that retains its aesthetic appeal even in harsh environments. This is particularly important for industries where the visual quality of components is a consideration, such as consumer goods, architecture, and medical devices.
3. Increased Safety and Environmental Friendliness
Citric acid passivation offers an environmentally friendly alternative to traditional nitric acid methods. Citric acid is biodegradable and poses fewer safety hazards, making it a safer choice for personnel and the environment. The safety benefits extend to the workplace, ensuring a healthier and more responsible environment. Additionally, by improving the corrosion resistance and durability of stainless steel, passivation reduces the need for frequent maintenance and replacement of corroded parts, minimizing waste and environmental impact.
Types of Equipment for Stainless Steel Passivation
Passivating stainless steel is a crucial process that enhances its corrosion resistance. The process requires specific equipment to optimize cleaning, rinsing, passivating, and final rinsing. Here are some types of equipment used for stainless steel passivation:

Small Benchtop Passivation Equipment: These manual passivation systems are ideal for areas with limited space and are generally used for passivating small parts and specimens.
Wet Bench Passivation Equipment: This integrated system resembles an assembly line. It provides ample space for all the passivation steps and includes safety features like in-tank flow and exhaust for nitric acid methods. This type of equipment is also manual.
Automated Passivation Systems: As the name suggests, these systems operate on the same principle as manual systems but eliminate the need for human intervention throughout the process. They are designed to provide a consistent and efficient passivation process.
Agitated Immersion Passivation Systems: These systems use agitation to enhance the passivation process. The agitation can help remove contaminants more effectively and ensure a uniform passivation layer.
In addition to these equipment types, specialized systems are designed for specific applications or industries. Some systems are designed for passivating large parts or high-volume production, while others are intended for cleanroom environments.
Regardless of the equipment used, it’s essential to maintain a careful balance of immersion time, bath temperature, and concentration to avoid damaging the stainless steel surface. Regular maintenance and equipment cleaning are also crucial to ensure effective passivation.
Applications of Passivated Stainless Steel
Pharmaceutical and Medical Devices
Passivation is critical in the pharmaceutical and medical industries for ensuring the cleanliness, reliability, and longevity of stainless steel tools and devices. Surgical tools benefit from passivation by maintaining their sharpness and being protected from rust and other forms of corrosion. This process is essential for medical devices as it helps to ensure they are free of impurities and can withstand the test of time while maintaining a smooth, clean surface that is easier to sterilize. The additional layer of protection from passivation increases the durability of medical instruments, allowing them to be used for extended periods without the risk of corrosion, which can cause instruments to rust. Passivation protects battery components, orthopedic implants, pacemakers, and other sensitive medical equipment.
Aerospace and Automotive Industries
Passivation is widely used in the aerospace industry to protect vital components such as turbine blades, instrument panels, and hydraulic components from contaminants. It improves the corrosion resistance of aircraft parts and fasteners, which is crucial for the safety and longevity of aerospace vehicles. In the automotive sector, passivation enhances the longevity of engine parts and fasteners, contributing to vehicles’ overall reliability and performance. The process ensures these components can withstand harsh environmental conditions and resist corrosion over time.
Food Processing and Electronics
In food processing, stainless steel equipment must remain hygienic and uncorroded to meet industry standards. Passivation services are employed for filters, screens, tubing, mixers, and storage tanks to ensure they stay free from rust and other contaminants. Passivation protects metal components like printed circuit boards from corrosion in the electronics industry, guaranteeing the reliability and longevity of electronic devices and circuitry. This is particularly important for maintaining the functionality of electronic devices and preventing failures due to corrosion-related damage.
Challenges and Considerations in Passivation
Factors Affecting the Effectiveness of Passivation
Several factors, including the stainless steel surface’s cleanliness, the metal’s composition, and the surface’s condition, influence the effectiveness of passivation. Contaminants such as iron must be removed to allow the chromium in the stainless steel to react with oxygen and form a protective chromium oxide layer. The temperature and pH of the passivation solution are critical parameters that must be controlled to ensure the process is effective. The passivation solution’s concentration and the passive layer’s thickness are essential for long-term corrosion prevention.
Environmental Concerns and Safety Procedures
Passivation involves the use of chemicals that can be hazardous, necessitating strict safety procedures to protect personnel and equipment. The environmental impact of these chemicals is also a concern, as improper management can lead to harmful effects. Compliance with environmental regulations and proper disposal of passivation chemicals can be complex and costly. Personal protective equipment (PPE), adequate ventilation, and proper storage and handling of chemicals are essential safety measures.
Comparison with Other Corrosion Protection Methods
Passivation is distinct from other corrosion protection methods, such as plating or using sacrificial anodes. While plating improves appearance and provides corrosion protection, passivation enhances the metal’s inherent resistance to corrosion without adding an external layer. Passivation relies on forming a passive oxide layer on the metal’s surface, which differs from coatings that add a separate protective layer. Unlike nitric acid passivation, citric acid passivation is environmentally friendly and does not produce toxic gases, making it a safer alternative.
Maintenance and Longevity: System Maintenance and Downtime Reduction, Long-Term Durability, and Cost Savings
System Maintenance and Downtime Reduction
Proactive maintenance strategies, such as regular inspections, cleaning, and timely repairs, are essential for minimizing system downtime and extending equipment life. By identifying and addressing potential failures early, companies can better plan and allocate resources, which leads to improved time management and reduced costs. Automated monitoring practices and predictive maintenance software can further ease the load on technical personnel and minimize troubleshooting time. Implementing strategic upgrades and regular maintenance updates can enhance system stability and reliability, reducing downtime. Proactive monitoring and regular backups are also crucial for the early detection of issues and ensuring minimal downtime during system recovery.
Long-term durability and Cost Savings
Proactive maintenance contributes to long-term durability and cost savings by reducing the need for frequent replacements and ensuring that facilities are reliable over time. This approach is more cost-effective than reactive maintenance, as it helps to minimize costly repairs and replacements. By focusing on the root causes of failures and taking proactive actions, proactive maintenance can lower total maintenance costs by as much as 60%. Regular upkeep ensures equipment operates at peak efficiency, reducing energy consumption and lowering expenses. Additionally, a well-maintained environment minimizes the risk of accidents and injuries, improving overall employee well-being.
Considerations for Implementing Proactive Maintenance
When implementing a proactive maintenance strategy, it is essential to consider the upfront investment in planning, time, expertise, and resources. Training maintenance staff and empowering employees to recognize signs of wear and instability are critical for supporting a preventative maintenance plan. CMMS, predictive analytics software, and IoT can streamline maintenance tasks and provide detailed insights for better decision-making. Regularly benchmarking and adapting maintenance procedures based on industry best practices and lessons learned is essential for continuous improvement.
Advanced Techniques in Passivation
Electrochemical Passivation
Electrochemical passivation, commonly known as electropolishing, is a highly effective method for enhancing the corrosion resistance of stainless steel. This process involves immersing the stainless steel components in an electrolyte bath and applying an electric current, which removes the outer layer of the material. Electropolishing improves the surface finish, providing a mirror-like appearance and removing imperfections such as micro-burrs and surface impurities that could harbor corrosion-initiating particles. Scientific studies have confirmed that electropolishing is superior to traditional chemical passivation methods in achieving passivation and enhancing corrosion resistance.
Innovations in Passivation Technology and Practices
Recent advancements in passivation technology have focused on reformulating the chemistry used in the process. The traditional use of mineral acids like nitric or phosphoric has been challenged by the introduction of organic acids, primarily citric acid, and other chelant materials. These new formulations are environmentally friendlier and have proven to be equal or superior to nitric acid passivation in effectiveness. Developing new test methods such as AES, XPS/ESCA, and Cyclic Polarization is revolutionizing how passivation is measured and validated. These methods directly measure corrosion rates, which can help design better treatment processes and schedule maintenance.
The Carpenter Technology Corporation has developed a specific alkaline-acid-alkaline (A-A-A) method that ensures optimal corrosion resistance for their stainless steel products. This method involves a thorough cleaning step followed by an acid bath, which can be nitric acid, nitric acid with sodium dichromate, or citric acid passivation. The A-A-A method is designed to neutralize trapped acid and is completed in less than two hours, making it a quick and efficient process.
LongSheng: The Perfect Manufacturing Partner for Stainless Steel Passivation
Outsourcing is always the better option for small and medium businesses regarding manufacturing services. You get access to top-of-the-shelf technology and work with experienced teams solving similar issues for many industries globally.
For any manufacturing service ranging from part production to passivation of stainless steel. LongSheng is easily among the top choices because of our commitment to quality and stringent standards that maintain consistent results.
It all starts with the suitable material and the right processes. We understand that and have an optimized system for stainless steel and all other metal parts. You’ll need to enhance your part’s durability and performance from stainless steel passivation to all the different surface finishing. Our team at LongSheng has the skills, experience, and knowledge to handle all your requirements and deliver results quickly.
Conclusion: The Future of Stainless Steel Passivation
Summarizing the Impact on Durability and Versatility
Stainless steel passivation is a critical process that significantly enhances the material’s durability and versatility. By creating a protective chromium oxide layer, passivation improves the metal’s resistance to corrosion and environmental conditions, making it more suitable for various applications. This process extends the lifespan of stainless steel components and enhances their aesthetic appeal, which is essential in industries where visual appearance is valued.
The future of stainless steel passivation looks promising, with ongoing research and development leading to innovations and exciting applications. Advancements such as nanotechnology are creating high-strength stainless steels with superior properties, while 3D printing is enabling the production of complex shapes and structures, offering reduced waste and faster prototyping. Additionally, PVD coating adds color and enhances the city, making stainless steel surfaces even more resistant to corrosion-resistance challenges such as the need for standardized design methods and more efficient production techniques; stainless steel continues to offer solutions to the demands of sustainability, durability, and aesthetics in construction and other industries. Passivation is crucial in ensuring the longevity and safety of vessels and structures in the marine sector, contributing to environmental sustainability by reducing the frequency of component replacements.
Passivation processes have become more environmentally friendly and practical, adopting citric acid as a safer alternative to traditional mineral acids. Best practices and industry standards have been established to ensure consistent quality and reliability in passivation.
As we look ahead, the versatility of stainless steel casting and advanced passivation techniques is set to expand further, delivering solutions that meet current demands and foster future innovations. Passivation remains a necessary step after fabrication to remove contaminants and enhance the corrosion resistance of stainless steel, ensuring the safety and longevity of products across various sectors.
In conclusion, the impact of stainless steel passivation on durability and versatility is profound and enduring. With continuous improvements in passivation technology and practices, stainless steel will remain an indispensable material, providing industries with reliable, long-lasting, and versatile components essential for progress and innovation.
FAQs
What is Stainless Steel Passivation?
Stainless steel passivation is a chemical process that removes excess iron from the material’s surface, resulting in an inert oxide layer that protects the steel from rusting. This process forms a protective layer known as the passive layer, which is only one to three nanometers thick. Despite its thinness, this layer protects against corrosion if conditions are right and remain stable.
Why is Passivation Necessary?
Passivation is necessary because it enhances the corrosion resistance of stainless steel, a critical element in many construction, manufacturing, transportation, and food processing applications. After machining, stainless steel components often have free iron left over that risks contaminating the workpiece. Passivation cleanses these contaminants and creates the necessary passive layer that makes stainless steel resistant to corrosion.
What are the Problems with Passivation of Stainless Steel?
Passivation of stainless steel can be problematic. Incorrectly performed passivation can induce corrosion. The process is sometimes misunderstood, and there is no universal agreement on the precise mechanics of how passivation works. However, a protective oxide film is sure to be present on the surface of passive stainless steel.
Does 304 vs. 316 Stainless Steel Need to be Passivated?
Passivation is recommended for both 304 and 316 grades for long service life. While 316 stainless steel does have better corrosion resistance due to its higher molybdenum content, it does not provide enough protection for most industrial environments.
How Often Should You Passivate Stainless Steel?
The frequency of passivation depends on the specific use case and environmental conditions. Chemical tests like the Ferroxyl test are often used to determine when passivation is necessary.
What are the Advancements in Passivation Technology?
Historically, nitric acid has been used to passivate stainless steel, but recently, a safer and more effective means of using citric acid has been introduced. Unlike nitric acid, citric acid passivation only removes iron and does not introduce heavy metals into the bath, making it much safer and environmentally friendly.
What is the Future of Stainless Steel Passivation?
The future of stainless steel passivation looks promising, with ongoing research and development leading to innovations. Advancements such as nanotechnology are creating high-strength stainless steel with superior properties, while 3D printing enables the production of complex shapes and structures. Additionally, PVD coating adds color and enhancesility, making stainless steel surfaces more resistant to wear and corrosion.


Pingback: Teflon Coating Stainless Steel: Durable PTFE Coatings
Thank you for your support and attention, we provide you with valuable content every day.
Thank you for your support and attention, we provide you with valuable content every day.
Pingback: 18/10 stainless steel vs 304
Pingback: 18/10, 18/8, and 18/0 Stainless Steel: What Are the Differences?
Hello.This article was extremely fascinating, especially because I was looking for thoughts on this subject last week.
Thank you for your attention, please subscribe to us and share valuable information with your friends in need, have any needs, please send us an inquiry.
I’d should examine with you here. Which isn’t something I normally do! I get pleasure from studying a submit that may make individuals think. Also, thanks for allowing me to remark!